Publications and Project Outcomes
This section highlights key publications, findings, and insights from the PICKED project, showcasing our contributions to chronic kidney disease (CKD) research and personalized medicine. Our work focuses on advancing biomarkers for early detection, developing models for disease progression, and exploring innovative intervention strategies to improve patient outcomes.
Our multidisciplinary team regularly publishes in high-impact journals and presents at international conferences, ensuring that our findings contribute to the broader scientific community. Stay tuned for the latest research updates and discoveries from the PICKED project!

Improving Haemodiafiltration through Smart Ultrafiltration Monitoring
We are excited to announce the latest publication from the PICKED project, led by RD Nephrologie SAS (RDN), now published in Scientific Reports under the title:
“In vitro evaluation of critical ultrafiltration fluxes and transmembrane pressure in a high flux dialyzer”
What’s the breakthrough?
High-volume haemodiafiltration (HDF) offers many benefits for patients with end-stage kidney disease. However, pushing for high convection volumes often creates practical complications — particularly pressure alarms and membrane clogging — which limit its use.
This new study evaluates the use of the GKD-UF max approach to define a critical and sustainable ultrafiltration rate. The research shows that:
- Stable and efficient ultrafiltration can be achieved by identifying the point of maximum filtration efficiency before fouling starts.
- The method helps prevent membrane clogging and reduces treatment interruptions, improving patient safety and dialysis quality.
- The approach can be implemented with existing online HDF machines, offering an accessible optimization path for dialysis centers.
Why is this important?
Haemodiafiltration is underused despite its benefits, partly because of the technical challenges it brings. This study offers a practical, data-driven solution to one of the key barriers: how to optimize ultrafiltration without causing damage or treatment failure.
Who contributed?
The research was conducted by Siavash Sohan Gir during his PhD at RDN within the PICKED MSCA Doctoral Network. It reflects the project’s mission to drive innovation in nephrology through interdisciplinary, patient-centered research and cutting-edge technology.
Acknowledgment
This work was supported by the European Union’s Horizon Europe Marie Skłodowska-Curie Actions (MSCA) under Grant Agreement No. 101168626 (PICKED project).
🔬 Stay tuned for more research updates from the PICKED network.
Follow us on LinkedIn, Bluesky and X for the latest news and publications!
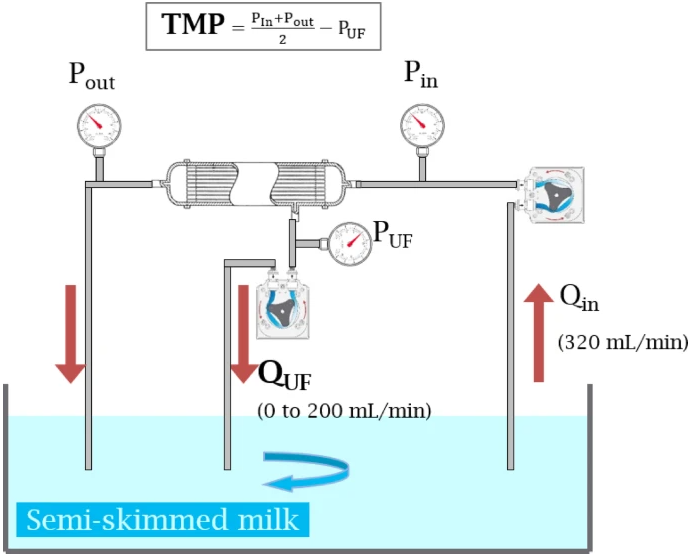
Diagram of the cross-flow filtration system used in all experiments.
Combination Therapy vs Biomarker-Guided Precision in Chronic Kidney Disease: Charting the Next Decade
Chronic kidney disease (CKD)—now affecting almost one billion people worldwide—demands therapies that can keep pace with its multi-factorial biology. In a new state-of-the-science review published in Biomolecules (Vol. 15, Article 809, June 2025), Sajjad Biglari, Harald Mischak and colleagues pose a decisive question: Should we rely on fixed-dose combination therapy (“polypill”) or move toward biomarker-guided personalised intervention?
Why this matters
CKD progression is driven by intersecting pathways—fibrosis, metabolic stress, haemodynamic overload and inflammation—so a single drug seldom suffices.
Modern “backbone” agents (SGLT2 inhibitors, non-steroidal MRAs, GLP-1 receptor agonists) already slow eGFR decline and curb cardiovascular risk.
Urinary-peptide classifiers (e.g., CKD273) now forecast renal or cardiac events years in advance, opening the door to precision escalation—or de-escalation—of therapy.
What the review highlights
Early combination regimens look powerful. Pilot trials that layer an SGLT2 inhibitor with finerenone (or add a GLP-1 RA) achieve > 50 % albuminuria reduction while mitigating hyperkalaemia risk. Yet cost and pill-burden remain hurdles.
Biomarker-guided treatment promises “right-patient-right-drug”. Risk classifiers can flag who is likely to benefit most from RAAS blockade, SGLT2i or MRA therapy, potentially sparing low-risk patients unnecessary exposure and expense.
A hybrid paradigm may win out. The authors envision a low-cost ARB + SGLT2i scaffold, fine-tuned by biomarker trends over time—a concept that dovetails with the PICKED network’s modelling work.
Read the full article
This research is supported by the PICKED project (HORIZON-MSCA-2023-DN-01, 101168626) and other European initiatives that are shaping the future of precision nephrology.

Summary of CKD progression, diagnosis, and respective treatment options.
Computational Drug Repositioning in Cardiorenal Disease: Advancing Therapeutic Innovation
Drug repositioning—the search for new therapeutic uses of existing drugs—has gained increasing momentum, particularly in tackling complex conditions like cardiorenal disease. While publications in this field continue to rise, the true measure of success lies in translating computational discoveries into real-world patient benefits.
We explore cutting-edge approaches in computational drug repositioning, leveraging bioinformatics, omics data integration, and network-based modeling to identify novel therapeutic opportunities. Despite significant progress, challenges remain in moving promising candidates from discovery to clinical application. Addressing these barriers with targeted strategies will be key to ensuring that repositioned drugs reach patients in need.
🔗 Read the full article here: DOI: 10.1002/pmic.202400109
This research is supported by the PICKED project (HORIZON-MSCA-2023-DN-01, 101168626) and other major European initiatives, driving forward the future of precision medicine.
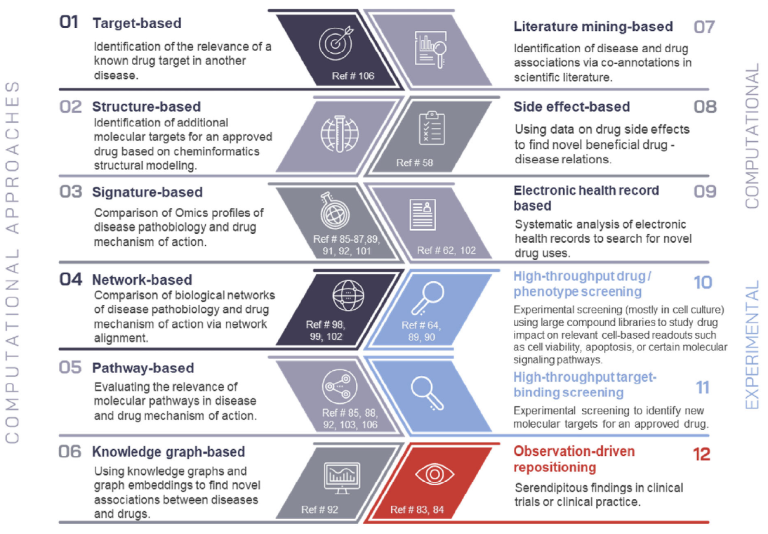
Overview on drug repositioning approaches.
Clinical Proteomics: From Scientific Breakthroughs to Patient Benefit
This article examines the progress and challenges of clinical proteomics, a field that has produced over 40,000 publications but has yet to make a widespread impact in clinical practice. Success should not be measured by publication volume alone but by tangible improvements in patient management and treatment.
We outline concrete solutions, emphasizing the need for a patient-centric approach to ensure that research efforts remain aligned with clinical utility. By addressing these barriers, we aim to shape the future of clinical proteomics, driving meaningful improvements in healthcare.
🔗 Read the full article here: DOI: 10.1002/pmic.202400346
This study represents a significant contribution to the field, supported by the PICKED project (HORIZON-MSCA-2023-DN-01, 101168626) and other major European research initiatives, driving innovation in personalized medicine.
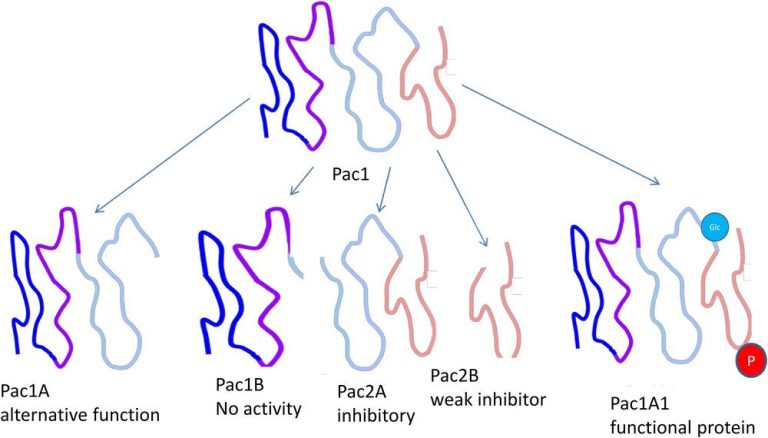
Biological functions of a hypothetical protein, Pac1, of which differently modified variants with different biological functions exist. The (hypothetical) protein Pac1 has little biological activity as a native polypeptide chain, but is converted into its active form, Pac1A1, by phosphorylation (red) and glycosylation (blue). The protein can also be specifically cleaved by proteases into Pac1A, which fulfils an alternative biological function, Pac1B which has no known function, and Pac2A and B, which inhibit the activity of Pac1A1.
Exploring the Role of GLP-1 RAs in CKD and Cardiometabolic Health
This article explores the potential of Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RAs) as transformative agents in the management of chronic kidney disease (CKD), type 2 diabetes (T2DM), obesity, and cardiovascular disease (CVD). These medications, including liraglutide, semaglutide, and tirzepatide, have shown significant benefits in metabolic control, weight reduction, and cardiovascular protection, making them an important consideration for nephrologists.
🔗 Read the full article here: https://pubmed.ncbi.nlm.nih.gov/39583142/
This marks an exciting milestone for the PICKED project, contributing valuable insights to the intersection of nephrology, metabolic health, and personalized medicine.
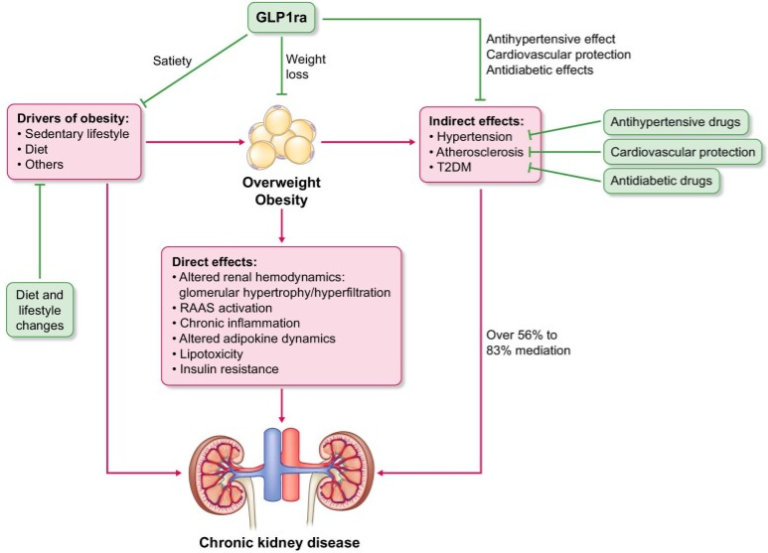
Obesity is an independent risk factor for CKD with a complex pathophysiology involving shared risk factors, direct effects of obesity and indirect effects through promotion of vascular disease, hypertension and T2DM. Indirect effects through BP and hyperglycaemia are estimated to mediate up to 83% of the negative impact of obesity on CKD. Mediation analyses of liraglutide and semaglutide in the T2DM CVOTs estimated up 48% mediation of kidney effects indirectly, through BP and hyperglycaemia. Similar analyses are awaited for overweight/obesity trials in which participants did not have T2DM.